Before starting with Class 7 Science Chapter 5 i.e. ‘Physical and Chemical Changes’, I am hoping that you have completed Chapter 4 of the 7th class. If not, then you can go through its Notes and NCERT Exercise Solutions whose links have been provided below. ⤵️
Table of Content
Introduction
Well, happy birthday in advance my children.

So, did you and your friends have fun inflating balloons, cutting the cake, and eating it, giving and receiving gifts?
I am hoping that you all had a wonderful time together. Now, let’s get back to our topic, shall we?
Every day we come across many changes around us and within us. Change is a part of our life.
Like in the above example, we inflated the balloons (they grew bigger in size), cut the cake into pieces and then ate it. Things change, some completely some tentatively.
Balloons grew bigger in size as we inflated them and they reduced back to almost their original size as we deflated them.
The cake that we cut into pieces was still a cake just its shape and size got changed.
A piece of cake that we eat after digestion doesn’t remain as cake, it has changed into something else now (faeces).
In Science, the changes have been divided broadly into two categories, physical change and chemical change.
Physical Change
To understand this concept in detail we’ll first go through some examples and activities that you may be able to perform at home.
ACTIVITY: 1
- Take a page out of your notebook.
- Now tear/cut it into four equal pieces (either square or rectangular).
- Lay these pieces onto the ground and try to recreate the shape of the original paper.
- Of course, you don’t know any sorcery to join the pieces back together and remake the original paper.
- But, the pieces of paper are still made of up paper. That hasn’t changed yet.
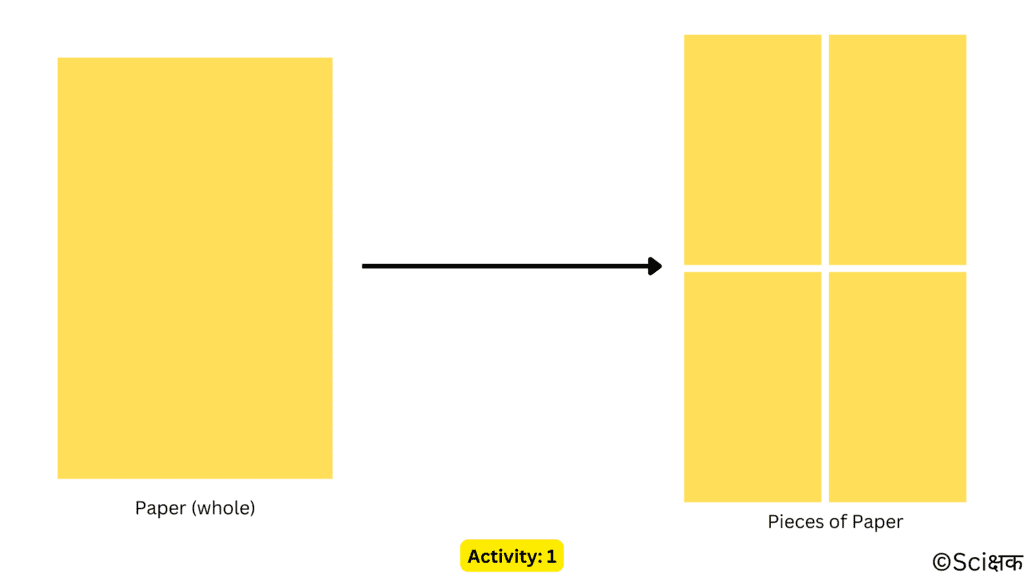
- Only the shape (may or may not be) and size of the original paper change when you tear it into pieces.
ACTIVITY: 2
- Take some ice in a vessel.
- And keep it in the Sun until all of it melts.
- Now once the ice has melted, refreeze it into a freezer to recover the ice.
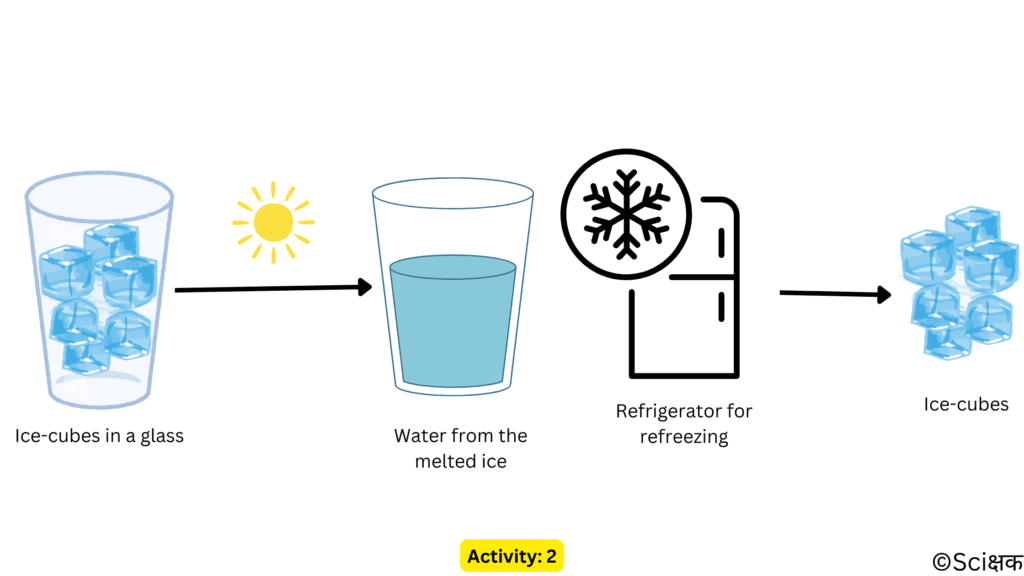
- Only the state of the water changed during this activity. (From ice to water to ice again).
ACTIVITY: 3
- Take an iron nail and hold it with a plier.
- Now heat the tip of the nail on a flame.
- Do this carefully or with the assistance of an elder,
- Look at the change in the tip of the iron nail.
- Once the iron nail has become red hot remove it from the flame and let it cool down and then again look at the tip of the iron nail.
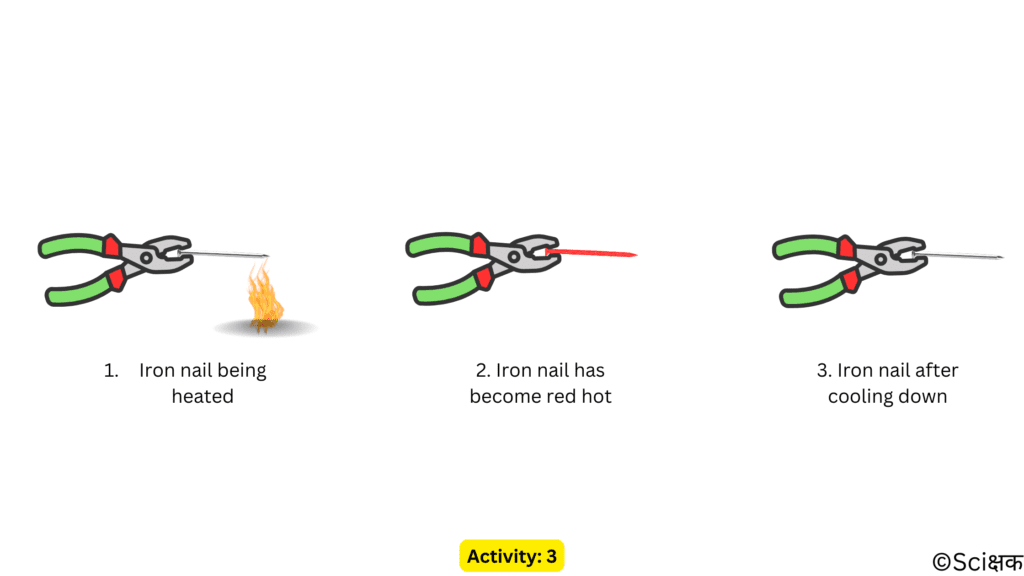
- The iron nail has acquired its colour back.
Properties such as the shape, size, colour, state of the substance, etc. are called its physical properties.
A change in the physical properties of a substance is called a physical change.
- No (chemically*) new substance is formed by a physical change.
- Physical changes are generally reversible but some can be irreversible as well.
We have mentioned the term ‘chemically’ above, well you will get to know about its basics further into the chapter and in detail you’ll learn in higher classes.
Chemical Change
Have you ever noticed that iron articles like gates, cars, scooters, benches, etc acquire a brown colour film or layer on them if left in the open?
Well, this brown colour substance is called rust and the process of layer formation is called rusting.
To understand the concept of chemical change in detail we’ll first go through some examples and activities (to be performed by the teacher or an expert).
ACTIVITY: 1
- Take a small piece of magnesium ribbon.
- Clean the tip of it with sandpaper.
- Then ignite the tip with a lighter or candle.
- Magnesium ribbon burns with a dazzling white flame.
- When it is completely burnt it leaves behind a white ash.
- Does this ash look like the magnesium ribbon? (No it doesn’t look like the magnesium ribbon, it’s different).
This change can be represented by the following equation:
Magnesium (Mg) + Oxygen (O2) ———–> Magnesium oxide (MgO)
This arrow in the equation implies ‘becomes‘.
Precaution: Don’t look at the burning magnesium ribbon for too long and don’t touch or taste the magnesium ribbon ash.
ACTIVITY: 2
- Collect the white dust in a beaker.
- Add some water to it and stir to make a solution.
- Now check its nature by using red and blue litmus paper.
- The solution is basic in nature as it turns red litmus to blue.
- The ash after mixing up with water forms a new substance or solution.
This change in the magnesium oxide can be represented by the following equation:
Magnesium oxide (MgO) + Water (H2O) ———–> Magnesium hydroxide ( Mg(OH)2)
Magnesium hydroxide forms by mixing magnesium oxide in water and we all know that it’s called ‘milk of magnesia’ and it is basic in nature.
ACTIVITY: 3
- Dissolve a teaspoon of copper sulphate (blue vitriol or neela thotha) in 100 mL of water in a beaker.
- Add a few drops of sulphuric acid to the solution and stir it till a blue colour solution is obtained.
- Now take out 10 mL of solution in a test tube.
- Now drop an iron nail into the solution and wait for a day or so.
- Observe the change in the colour of the solution.
- Compare the colour of the beaker with the colour of the solution in the test tube.
- Check the colour of the nail as well.
The colour of the solution in the beaker changes from blue to green. And, there is a brown deposit on the iron nail i.e. of copper.
The change that took place in the beaker can be written by the following equation:
Copper sulphate solution (blue) + Iron ———> Iron sulphate solution (green) + Copper (the brown deposit on the nail)
ACTIVITY: 4
- Take a teaspoon of vinegar (acid) in a test tube and add a pinch of baking soda (base) to it.
- You would hear a hissing sound and see bubbles of gas forming or coming out of the test tube.
- With the help of a pipe pass this gas through a freshly prepared lime water solution or slacked lime.
- Do you see any colour change in the lime water?
- The colour of the lime water turns milky as we pass the gas (carbon dioxide) through it due to the formation of calcium carbonate.
The change in the test tube can be described by the following equation:
Vinegar (Acetic acid) + Baking Soda (Sodium hydrogen carbonate) ———–> Sodium acetate (salt) + Water + Carbon dioxide
The reaction of carbon dioxide with lime water can be represented by the following equation:
Carbon dioxide (CO2) + Lime Water ( Ca(OH)2) ————> Calcium Carbonate ( CaCO3) + Water( H2O)
- Turning lime water milky is a standard test that is used to check for the presence of carbon dioxide.
A change in which one or more new substances are formed is called a chemical change or a chemical reaction.
Chemical changes are really important in our lives. All the new substances that are formed are results of chemical changes. e.g. digestion of food in our body, burning of fuel, ripening of fruits, fermentation of sugar, etc.
Useful new materials such as plastics, medicines, detergents, soaps, etc. are a result of chemical changes.
In addition to the formation of new products, the following may also accompany a chemical change:
- Heat, light or any other radiation (UV, IR, X-ray, etc.) may be given off or absorbed.
- Sound may be produced.
- A change in smell may take place or a new smell may be given off.
- A colour change may take place.
- A gas may be formed, etc.
Some more common examples of chemical changes:
- An explosion of a firework. It produces heat, light, sound and an unpleasant gas that is harmful for our atmosphere. That is why it is advised not to play with the fireworks.
- Cutting an apple into slices and after some time the slices acquire a brown colour.
- Neutralisation reactions.
- Photosynthesis and respiration.
- Conversion of Ozone (O3) —-> Oxygen (O2) in the atmosphere by absorbing UV radiations.
Did you know?
The ozone layer is present in our atmosphere’s stratosphere which protects us from harmful UV radiations. It absorbs the UV radiation and breaks down into the molecular oxygen. If ozone doesn’t filter out the UV radiation and let it reach the Earth’s surface, we can get severe radiation burns and skin cancer due to UV exposure.
Rusting of Iron
The process of rusting can be represented by the following simple equation:
Iron (Fe) + Oxygen (O2, from the air) + Water (H2O) ——–> Rust (Iron oxide, Fe2O3)
For an iron article to get rusted the presence of both water and air is important. Air or oxygen is present in fixed amounts in our atmosphere, but moisture or water content can vary so the more the water, the faster the rusting.
How to Prevent the Objects of Iron from Rusting
There are various methods and techniques that we can employ to prevent our iron objects from rusting. Here are some of these methods:
- By applying a coat of paint or grease to prevent the iron articles come in contact with water and air directly.
- Deposit a layer of another metal like zinc or chromium on the iron objects. The process of depositing a layer of zinc on the iron is called galvanisation.
- We can use stainless steel instead of iron for making objects. Steel is an alloy of iron and carbon and some other metals like chromium, nickel and manganese. Stainless steel is rust-proof.
Crystallisation
In the previous classes, we have learnt that salt can be obtained from the sea by evaporating the seawater.
But there is a small problem with the salts obtained by this method: they are not pure enough and the shape of their crystals can’t be seen clearly.
The process of formation of pure crystals of substances from their solutions is called crystallisation.
Now, let’s learn to prepare the pure crystals of copper sulphate (CuSO4)
Preparing the Pure Crystals of Copper Sulphate (CuSO4):
- Fill a beaker 3/4 with distilled water and add a few drops of dilute sulphuric acid to the beaker.
- Heat the beaker till the water starts to boil.
- Now, add copper sulphate powder slowly into the beaker with a constant stirring.
- Continue adding copper sulphate powder till the solution saturates.
- Now filter the solution through a filter paper and allow it to cool down.
- Don’t disturb the solution when it’s cooling.
- Look at the solution after some time, you will be able to spot or see some really beautiful blue-coloured copper sulphate crystals.
Conclusion
Our life, our thinking, our behaviours, our habits everything is defined by chemicals and changes in our body with time or events/experiences. Change is a part of life and change leads to the growth of a person.
Just like Dr. Marie Curie said “Nothing in life is to be feared, it is only to be understood. Now is the time to understand more, so that we may fear less.”
Understand and embrace the changes within yourself.
Credits & References
- Class 7th Science NCERT Textbook
- Image by Alexa from Pixabay
- Image by Myriams-Fotos from Pixabay
Thank You for Choosing Sciक्षक ❤️

