Before starting with Class 7 Science Chapter 4 i.e. ‘Acids, Bases and Salts’, I am hoping that you have completed Chapter 3 of the 7th class. If not, then you can go through its Notes and NCERT Exercise Solutions whose links have been provided below. ⤵️
Table of Content
Introduction
In the previous chapter, we learned about the buccal cavity and specifically about the tongue.
It is a fleshy muscular organ that is attached at the back to the floor or the buccal cavity. It is free at the front and can be moved in different directions.

The tongue has small buds (taste buds) present on it that are sensitive to various tastes and because of it, we can experience the sweet, bitter, sour, and salty nature of a food item.
Acids & Bases
Things like lemon, orange, pineapple, and curd, are sour in taste because they contain acids in them. So, their chemical nature is acidic.
The word ‘Acid‘ comes from the Latin word acere which means sour.
Acids that occur in natural entities like fruits, vegetables, plant secretions, animals, animal products, etc. are called natural acids.

Some of the common natural acids are:
- Formic acid is found in a bee and ant’s sting.
- Citric acid is found in citrus fruits like oranges, lemons, etc.
- Lactic acid is found in curd.
- Oxalic acid is found in spinach.
- Ascorbic acid is found in amla (Indian gooseberry)
- Tartaric acid is found in tamarind, grapes, etc.
Acids that are synthesized in laboratories are called synthetic acids.
Some of the common synthetic acids are:
- Sulfuric acid
- Hydrochloric acid
- Nitric acid
- Carbonic acid, etc.
Things like baking soda, neem leaves, etc. that are bitter in taste and soapy in feel when rubbed between the fingers are bases. So, their chemical nature is basic.
Bases that occur in natural entities like fruits, vegetables, plant secretions, animals, animal products, rocks etc. are called natural bases.

Some of the common natural bases are:
- Lime (calcium oxide)
- Neem leaves
- Limestone
- Carbonates present in water, etc.
Bases that are synthesized in laboratories are called synthetic bases.
Some of the common synthetic bases are:
- Sodium hydroxide
- Baking soda
- Bleaching powder
- Milk of magnesia (magnesium hydroxide)
- Window cleaner (ammonium hydroxide), etc.
Acid-Base Indicators
These are the special types of substances that are used to test whether a substance is acidic or basic in nature.
When added to a basic or an acidic solution, the indicators either change their colour or odour.
Acid-base indicators can be natural or synthetic in nature.
Some of the common naturally occurring acid-base indicators are:
- Litmus
- Turmeric
- China Rose or Hibiscus
- Red cabbage
- Clove
- Onion
- Beetroot, etc.
Some of the common synthetic acid-base indicators are:
- Phenolphthalein
- Methyl orange, etc.
Now, we’ll get to know about some of these indicators in a bit more detail.
1. Litmus: a natural dye
This is the most commonly used acid-base indicator. It is extracted from lichens.
When extracted from the lichens it has a mauve (purple colour). So, when added to an acidic solution it turns red. And, when added to a basic solution it turns blue.
There is a small word trick to remember the litmus paper colour changes.
- ABR —> Acid turns only Blue Litmus to Red. It doesn’t change the Red Litmus into Blue.
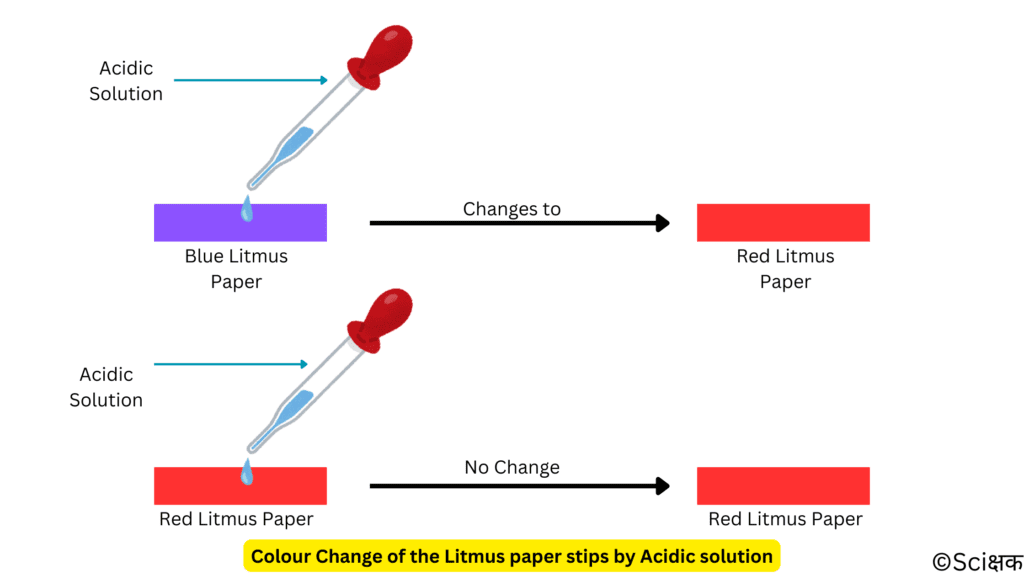
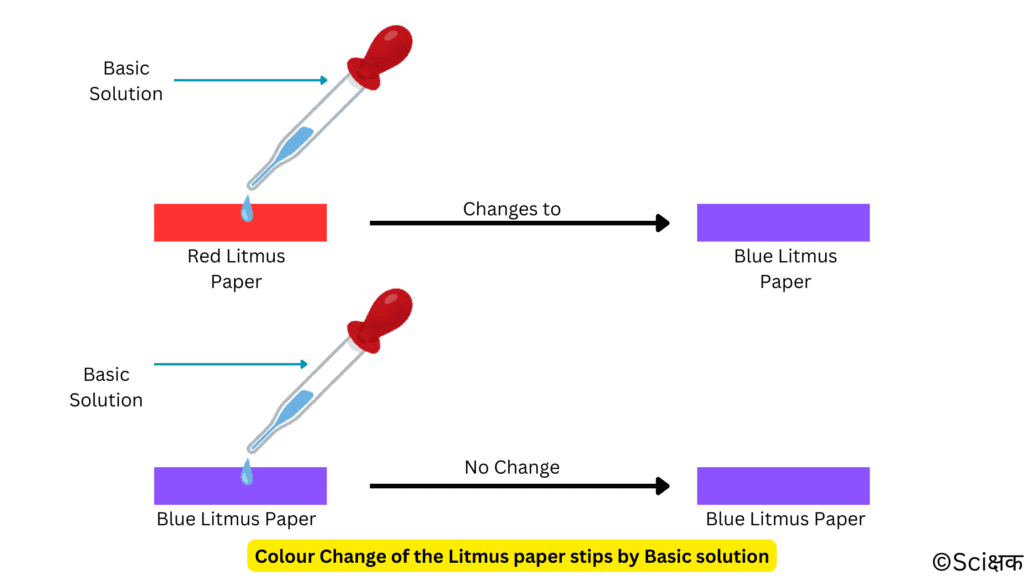
- BRB —> Base turns only Red Litmtus to Blue. It doesn’t change the Blue Litmus into Red.
Litmus is available in the form of both a solution and paper strips.
Earlier we used to use red or blue litmus paper for getting to know about the acidic or basic nature of a substance but in modern times we use Yellow coloured pH paper which can easily differentiate between an acidic or a basic solution saving us both time and resources.
The solutions that do not change the colour of either blue or red litmus are known as neutral solutions as they are neither acidic nor basic.
2. Turmeric
Yes, you read it right. Turmeric aka haldi, is a common Indian household spice.
It shows different colours in acidic and basic solutions.
- In basic solutions the yellow colour of turmeric changes to red colour.
- But, in an acidic solution, the yellow colour of turmeric doesn’t change.
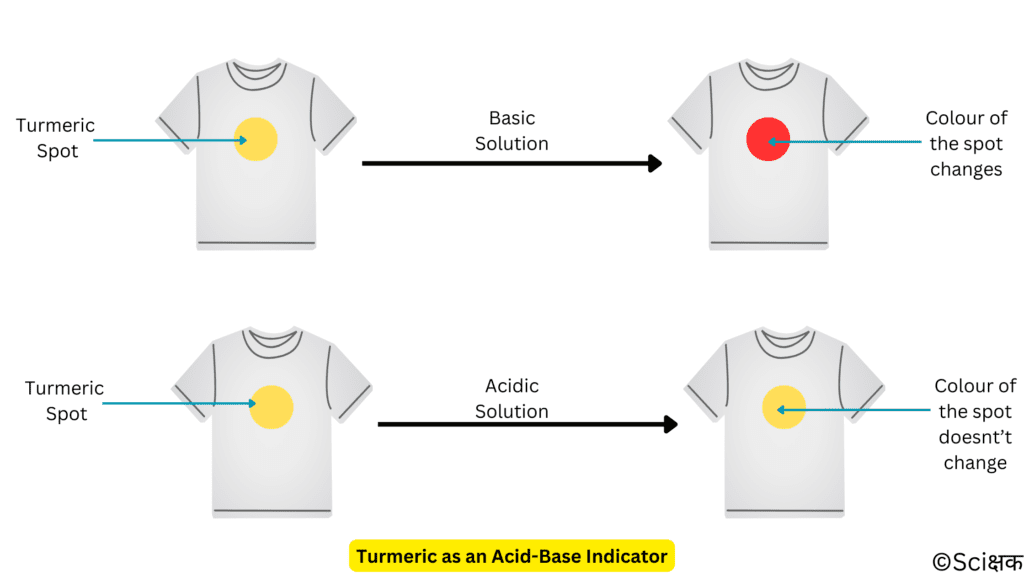
The soap that you use for washing your clothes is basic in nature and it changes the yellow turmeric stains on your white shirt to reddish in colour.
3. China Rose or Hibiscus Petals
China rose petals or their solution acts as an acid-base solution.

Preparing china rose petals solutions:
- Collect some china rose petals and place them in a beaker.
- Add some warm water to the beaker.
- Keep the mixture for some time till the water becomes coloured.
- Use this coloured water as an indicator.
It shows different colours in acidic and basic solutions.
- In an acidic solution, the light-pink colour of the china rose solution turns dark pink (magenta).
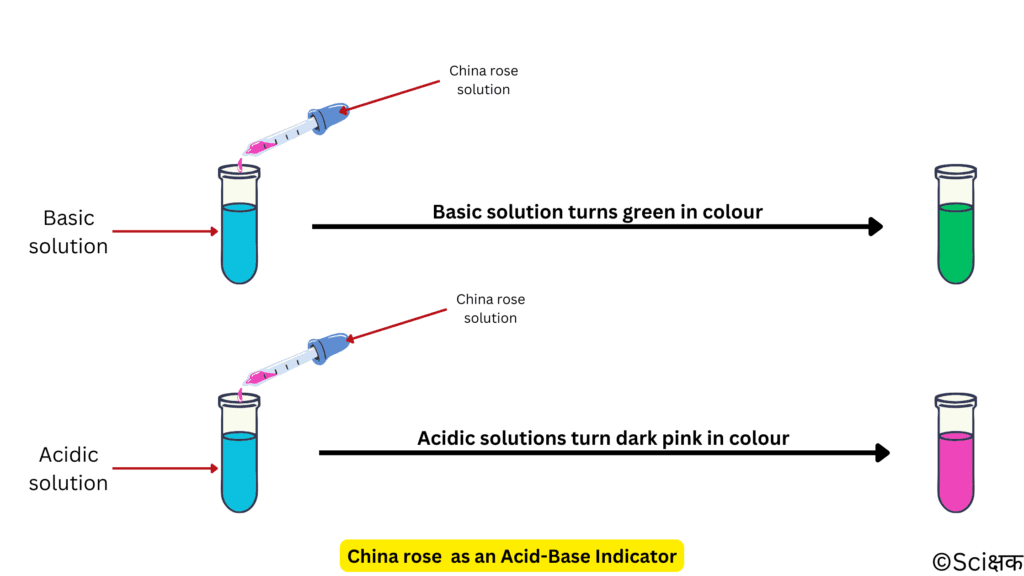
- In a basic solution, the light-pink colour of the china rose solution turns green.
Did You Know?
Coffee beans or powder is acidic in nature even though it’s bitter in taste.
4. Phenolphthalein
It is a synthetic acid-base indicator.
- In an acidic solution, it remains colourless.
- Whereas, in a basic solution it turns pink in colour.
It is mainly used in an acid-base neutralisation reaction to find out the end-point (when all acid has been neutralised by the base present in the solution mixture).
Neutralisation
When we mix an acidic solution with a basic solution the neutralisation reaction takes place producing salt and water.
An acid and a base are anti of each other meaning they cancel out each other in a solution. So if we mix hydrochloric acid (HCl) and sodium hydroxide base (NaOH) together, they will react together and produce salt (Sodium chloride, NaCl) and water (H2O).
Hydrochloric acid + Sodium hydroxide ————-> Sodium chloride + Water + heat
HCl + NaOH ———-> NaCl + H2O + heat
In a neutralisation reaction heat is released meaning that if we mix some hydrochloric acid and sodium hydroxide in a beaker, the beaker will get hot after some time.
Neutralisation in Everyday Life
Neutralisation reactions are not only limited to the laboratories but they are also really important for us in our daily life. Here are some of the most common examples of such important applications of neutralisation reaction:
1. Relief from Indigestion and Acidity
Have you ever felt a burning sensation in your stomach, followed by a bit of sour (acidic) burps?
In the previous chapter, we got to know about digestion in human beings and in that, we learnt about how food is digested in our stomach with the help of stomach acid or hydrochloric acid. But, sometimes our stomach secretes too much acid or the food that we are having may be a bit more acidic, increasing the acidity of stomach content to more than the suitable range causing a burning sensation in our stomach or indigestion.
To get relief from this increased acidity we take antacids (they are the substances that have anti effect of an acid, simply put bases).
Some of the commonly used antacids are baking soda, milk of magnesia, etc.
ENO is a very common antacid that is quite prevalent in our Indian households.
2. Ant’s Bite and Honeybee’s Sting
Have you ever been stung by a honeybee or bitten by ants?
After a bite or sting your skin becomes red and swells up due to the insertion of formic acid in your body by the honeybee(s) or ant(s).

To get relief from the swelling and pain you can apply wet baking soda (sodium hydrogen carbonate) or calamine (zinc carbonate) over the sting or bite mark.
Did You Know?
A wasp sting is basic in nature and to get relief from the pain and swelling you can apply an acid like lemon juice over the sting mark.
3. Soil Treatment
Each crop has a specific soil pH (indicates how acidic and basic soil is) requirement to grow optimally. So, it’s really important to maintain the acidity or basicity of soil according to the crop.
If the soil is:
- Too acidic then it can be treated by sprinkling lime or slaked lime solution in the fields.
- Or, if it is too basic then it can be treated by adding compost in the fields. Organic matter releases acid that neutralizes the basic soil.

So, it is really important for a farmer to get to know about his/her field’s pH.
4. Factory Wastes Treatment
Most of the water pollution is caused by industries dumping their untreated waste into the waterbodies killing millions of organisms including humans as well.

The industrial waste or factory waste is highly toxic, acidic or basic. It has to be treated, it has to be neutralised before dumping it into the water bodies.
Conclusion (Acid Rain)
Individually these two words may make sense to you. But together, what is this all about?
Well, it’s our human creation, our abomination.

Due to too much air pollution and release of pollutants like sulpher dioxide, nitrogen dioxide and carbon dioxide into the atmosphere. Our atmosphere has gotten polluted and the rain which is supposed to be neutral has gotten acidic due to the formation of carbonic acid, sulfuric acid and nitric acid in the atmosphere or clouds. When this acidic rain falls on the surface it harms the crops, people, organisms, soil and every living soul. Monuments that are made up of marble like the Taj Mahal have started to deteriorate.
And the situation is getting worse day by day. Two very simple and effective solutions to this problem are:
- Plant more and more trees.
- Use public transport more than private vehicles.
References & Credits
- Class 7th Science NCERT Textbook
- Image by StockSnap from Pixabay
- Image by MYCCF from Pixabay
- Image by punnamjai from Pixabay
- Image by Pexels from Pixabay
- Image by edisonjimenez10 from Pixabay
- Image by David Hablützel from Pixabay
- Image by Ajaya khadka from Pixabay
Thank You for Choosing Sciक्षक ❤️

