Before starting with Class 7 Science Chapter 3 i.e. ‘Heat’, I am hoping that you have completed Chapter 2 of the 7th class. If not, then you can go through its Notes and NCERT Exercise Solutions whose links have been provided below. ⤵️
Table of Content
Introduction
Have you ever noticed that we wear dark-coloured coats and sweaters in winter, and we wear light-coloured cotton clothes in summer?
In winter we feel cold inside our house and when we come out in the sun, we feel warm whereas in summer we feel hot inside our house.
So, how do we come to know whether an object or surrounding is hot or cold?
We’ll try to answer all these questions further in this chapter.
Hot and Cold
In winter we prefer to drink hot tea whereas in summer we prefer to drink cool lemonade.
We can find out whether an object is hot or cold by touching it with our skin (hand).
But there are a few problems with our sense of touch’s reliability:
- Sometimes the object may be too hot or cold that it can damage our skin like hot burn, frost bites etc.
- Second, our sense of touch is not reliable enough to tell whether an object is absolutely hot or cold.
We can prove this lacking in our sense of touch with a very simple activity that you can perform at home.
Activity to Check the Reliability of Our Sense of Touch
- Take three buckets or mugs and label them ‘A’, ‘B’ and ‘C’ respectively.
- Now pour cold water into bucket ‘A’ and hot water into bucket ‘B’.
- And Pour an equal amount of hot and cold water into bucket ‘C’.
- Now dip your left hand in bucket ‘A’ and right hand in bucket ‘B’ for 5 minutes.
- Now take out both of your hands and dip them into bucket ‘C’.
- Do both of your hands get the same feeling?
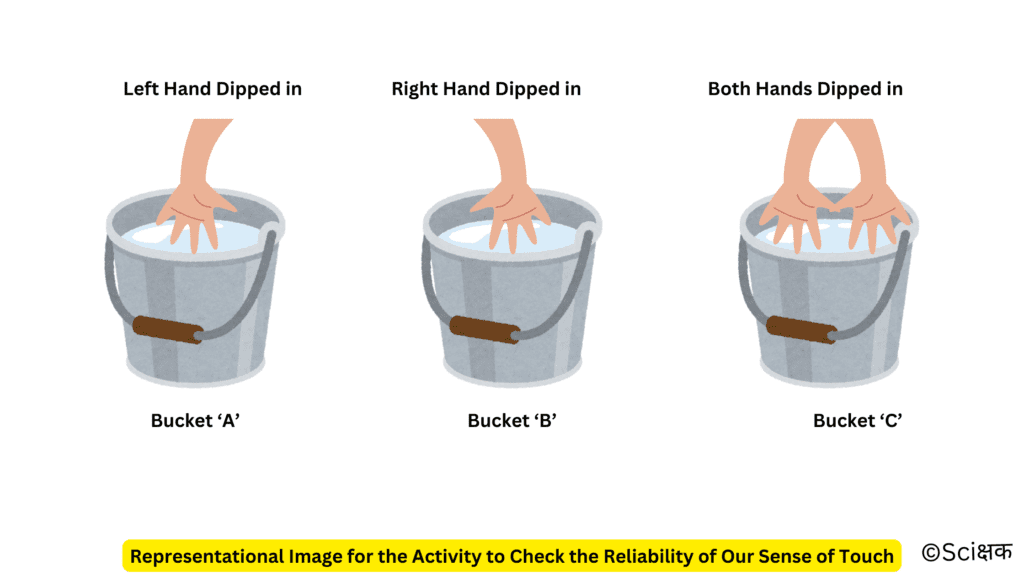
No, both of our hands will get a very different feeling. Our left hand will feel warm or hot in bucket ‘C’ whereas our right hand will feel cold in bucket ‘C’ even though the water in bucket ‘C’ is at the same temperature.
Our sense of touch can be deceiving sometimes that’s why we need something or some measure that is reliable in nature and for that we use temperature.
Temperature: is a measure of the degree of hotness of an object.
The device that we use to measure temperature is called a thermometer.
Measuring Temperature with a Thermometer
Have you ever had a fever and went to a doctor and the doctor measured your body temperature with a small glass tube-like thing that the doctor put into your mouth under your tongue?
Well, that was a clinical thermometer used to measure the body temperature of a person.
Clinical Thermometer
It is a medical device that is used to measure a person’s body temperature.
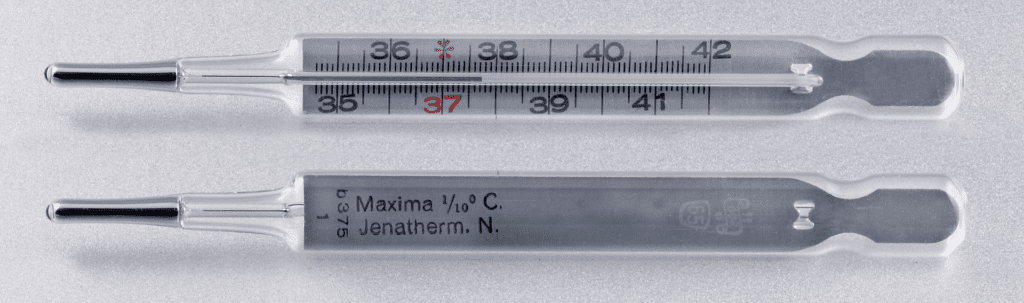
It consists of a long, narrow, uniform glass tube that has a mercury-filled bulb at one end. Outside the bulb, a small shiny thread of mercury can be seen. The glass tube is marked with a scale ranging from:
35°C – 42°C or 94°F – 108°F ( C = Celcius and F = Fahrenheit)
In India, we use the degree Celcius unit of temperature.
Reading a Thermometer
To read a thermometer accurately we have to follow a proper procedure.
Understanding the Limits, Range and Accuracy of a Thermometer
We have to first note down the difference indicated between two bigger marks and then we have to note down the number of divisions (no. of smaller marks) present between these two bigger marks to find out the value of each division so that we can measure the temperature more accurately.
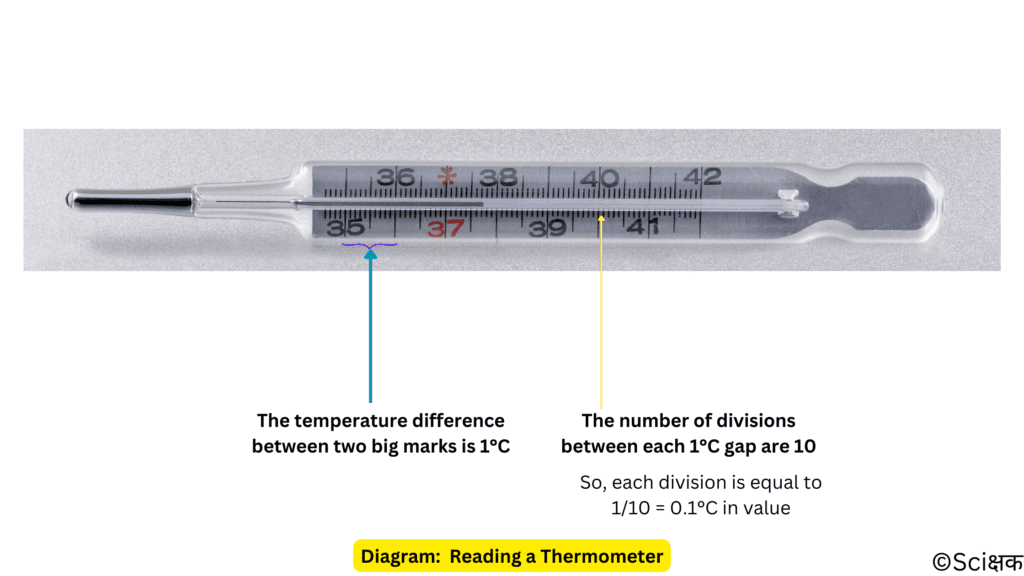
Let’s read a thermometer now:
- Before using a thermometer, wash it with an antiseptic solution like rubbing alcohol.
- Hold it firmly from the back and give it a few shakes to bring the level of mercury down.
- Ensure that the level of mercury falls below 35°C.
- Now place the thermometer under your tongue or under your armpit for a minute or two.
- Take the thermometer out of your mouth and note the readings in your preferred unit (degree Celcius or degree Fahrenheit).
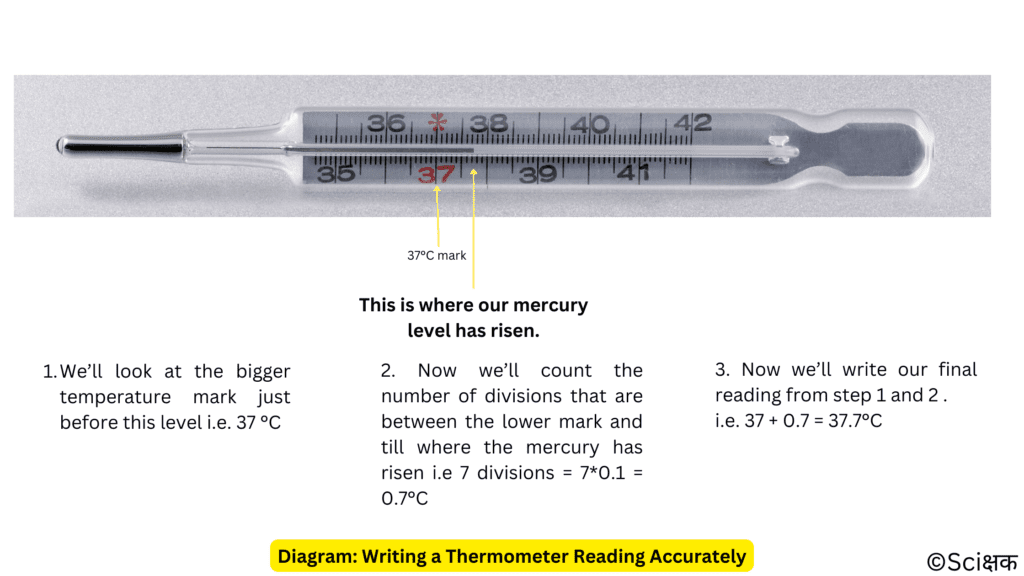
Let’s find out the reading(s) of the thermometer that we used in the previous picture.
Precautions to Keep in Mind while Using a Clinical Thermometer:
- Sterilize the thermometer with an antiseptic solution before using it.
- Don’t bite on the thermometer when it is placed under your tongue.
- Ensure that before using the thermometer the mercury level is below 35°C.
- Handle the thermometer with care, as it is made up of glass it can break easily if hit against any hard surface.
- Don’t hold the thermometer by the bulb while reading it.
- Don’t try to heat or put a clinical thermometer in flames or sunlight.
Normal Human Body Temperature
The average human body temperature is 37°C. From person to person it may vary slightly. 37°C temperature is actually the average body temperature of a large number of healthy populations.
Normally the internal temperature of the human body doesn’t fall below 35°C or rise above 42°C.
But if it falls under 35°C, a hypothermic condition occurs which can be fatal to human health.
A Laboratory Thermometer
To measure the temperature of objects and bodies other than the human body we use a laboratory thermometer.
It is also made up of a long, narrow, uniform glass tube filled with mercury.
The range of a laboratory thermometer is generally from -10°C to 110°C.
The procedure to measure and read the temperature using a laboratory thermometer is similar to that of a clinical thermometer.
Precautions to Keep in Mind while Using a Laboratory Thermometer:
- It should be kept upright not tilted.
- The mercury bulb of the thermometer should be surrounded from all sides by the substance of which the temperature is to be measured.
- The bulb should not touch the surface of a container.
The temperature on a laboratory thermometer drops quite fastly so it has to be read while the thermometer is in the liquid or object whose temperature we are measuring.
Why Can’t We Use a Laboratory Thermometer to Measure Our Bodily Temperature?
While measuring our body temperature, we have to take the thermometer out of our mouth or armpits to note the readings. We can’t use a laboratory thermometer for measuring our body temperature because the moment we take it out of our mouth, the mercury level will fall immediately giving us a wrong measurement.
In a clinical thermometer, we don’t have this issue because a clinical thermometer has a kink or constriction in it that prevents the rapid fall in the level of mercury.
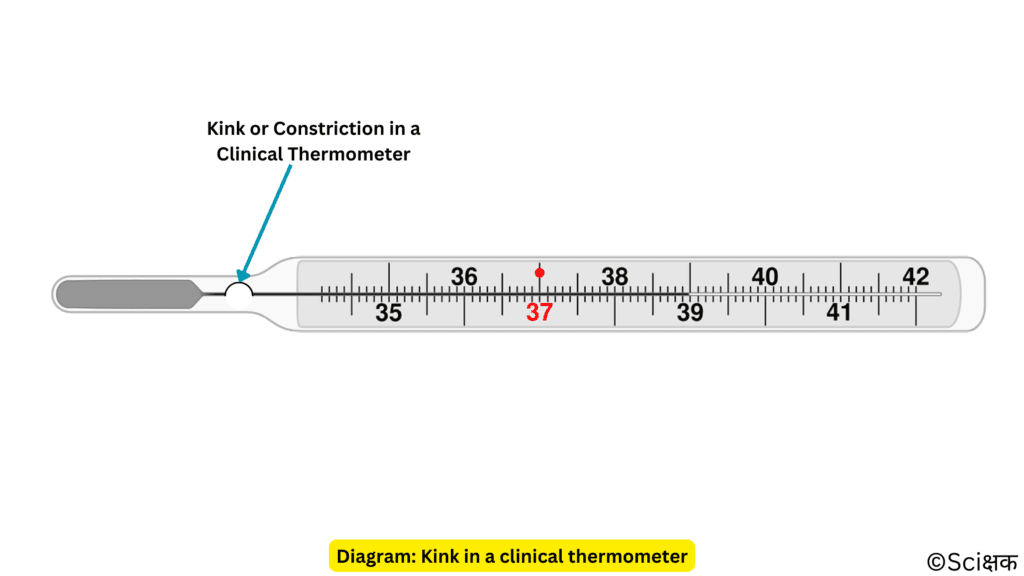
Here is a video that explains the working of kink in a clinical thermometer:
https://www.youtube.com/watch?v=44h8fIGqF6g (© Jay Physics YouTube Channel)
Digital Thermometers
Mercury is very toxic for human health so to prevent the ill effects of mercury we have started to use digital thermometers which don’t use mercury.

Transfer of Heat
Have you noticed that the frying pan becomes hot when kept on a flame?
This happens because the heat passes from the flame to the utensil and when the pan is removed from the stove, the pan cools down over a period of time. The heat was transferred from the pan to the surroundings.
Heat always flows from a hotter object to a colder object.
The mode of heat transfer may vary from object to object. Here are some of the common modes of heat transfer.
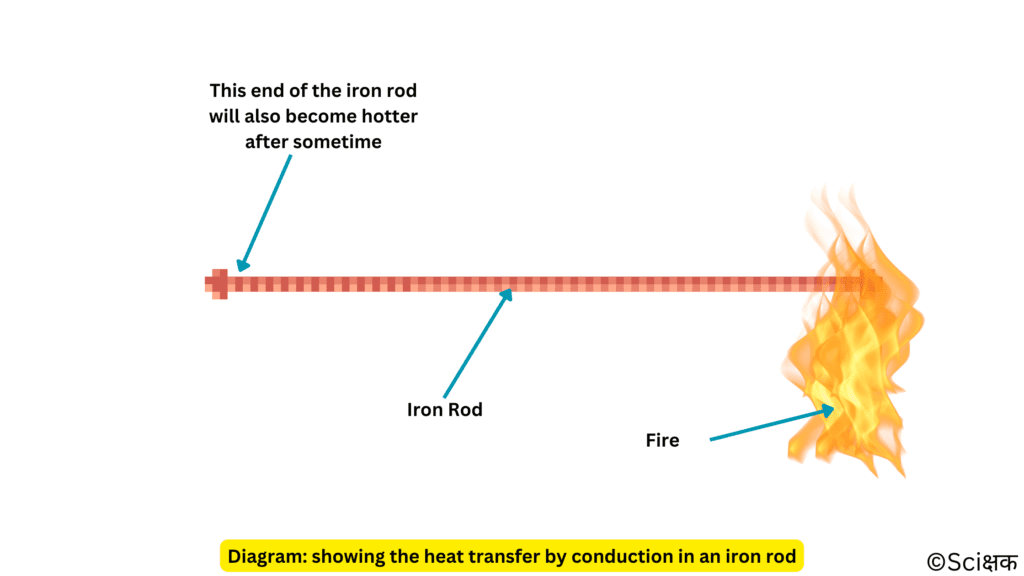
1. Conduction: mode of heat transfer in solids
In this mode the heat transfer from the hotter end of an object to the cooler end of the object. In solids, the heat is generally transferred by this mode.
You can test this by putting one end of a long iron rod in fire and slowly with time the whole rod will become hotter.
Not all solids conduct heat equally.
The materials that allow heat to pass through them easily are called conductors of heat. e.g. aluminium, iron, copper, gold, etc.
The materials that don’t allow heat to pass through them easily are called poor conductors or insulators of heat. e.g. wood, plastic, air, water, etc.
2. Convection: mode of heat transfer in air and water/liquids
This mode of heat transfer is quite common in air and water (liquids). They are a bad conductor of heat.
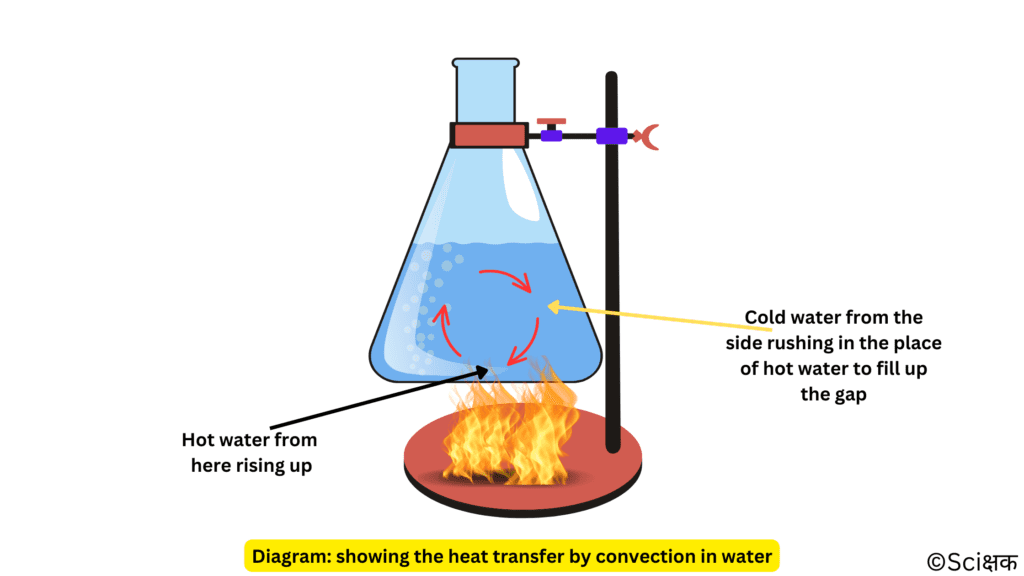
- When water is heated, then the water near the flame gets hot.
- Then this hot water rises up and the cold water from the sides moves down toward the source of heat.
- This water also gets hot and rises up and the water from the sides moves down.
- This process continues till the whole water gets heated.
- This mode of heat transfer is known as convection.
The same pattern of heat transfer is followed in air as well. The air near the source of heat gets hot and rises. The air from the sides comes in to take its place and in this way, the air gets heated.
Due to convection, an interesting phenomenon is observed in the coastal regions:
Sea Breeze: During the day the air over the land becomes hot and rises up, and the cooler air from the sea rushes in to take its place. The warm air from the land moves toward the sea to complete a cycle.
To receive the sea breeze, the windows of the houses on the coast are made to face the sea.

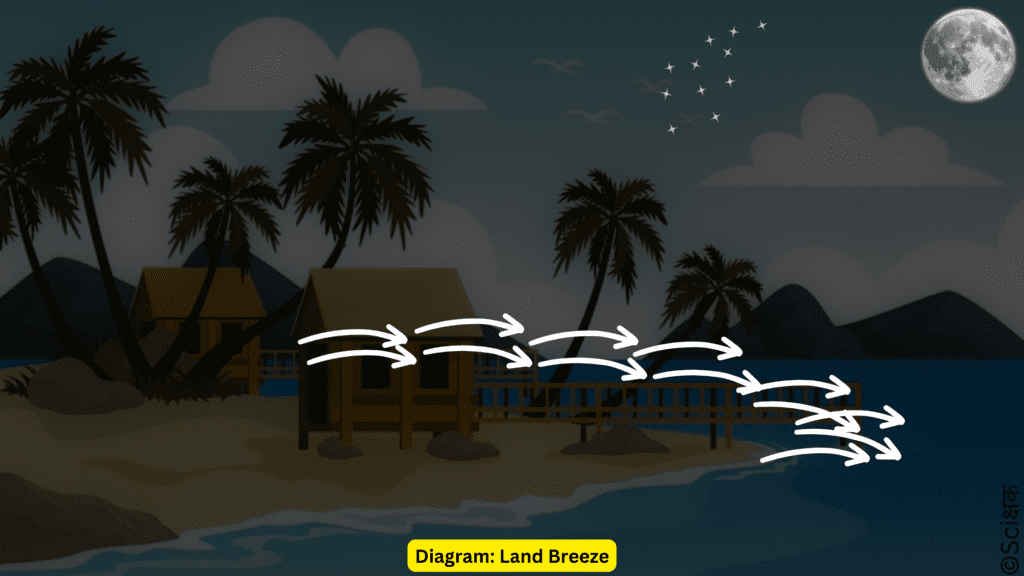
Land Breeze: At night, the exact reverse happens. The water cools more slowly than the land, so the hot air over the sea is replaced by the cooler air that flows from the land to the sea.
3. Radiation: mode of heat transfer in vacuum and in space
Have you ever wondered how the heat from the Sun reaches us here on Earth without the presence of any medium in between?
The heat from the sun reaches us by radiations that don’t require any medium to transfer.
An object (if hot) can lose heat to the surroundings (cold) via radiation and vice-versa.
All hot bodies radiate heat and when this heat falls on an object some of it gets reflected, some of it gets absorbed by the object and some is transmitted.
- When an object loses heat it becomes cooler.
- And, when an object absorbs heat it becomes warmer.
Relation of the Colour of an Object to The Amount of Heat Absorption
Black colour or black objects are said to be good absorbers and radiators of heat.

That is why we are advised to wear light colour clothes in summer and dark colour cloth in winter. As the dark colour cloth will absorb more heat or sunlight and we’ll feel warmer and comfortable. On the other hand, the light colour clothes will reflect most of the sunlight, keeping us cool and comfortable in summer.
Why do woollen clothes keep us warm in winter?

Wool is a poor conductor of heat because there is air trapped between the wool fibres. This air acts as an insulator and prevents the flow of heat from the body to the cold surroundings in winter. That’s why we feel warm in woollen clothes.
Conclusion
Fun Fact: Human beings are warm-blooded animals meaning their internal body temperature remains constant and doesn’t change with the change in the surrounding temperature. Our body performs a whole lot of functions to keep it constant e.g. sweating in summer, shivering when we feel cold, etc.
References & Credits
- Class 7th Science NCERT Textbook
- https://commons.wikimedia.org/w/index.php?curid=22416072
- https://en.wikipedia.org/wiki/Medical_thermometer
- Image by OpenClipart-Vectors from Pixabay
- Image by Clker-Free-Vector-Images from Pixabay
- Image by freepik
- Image by freepik
- Image by Victoria from Pixabay
- Image by Ben Kerckx from Pixabay
- Image by Gylfi Gylfason from Pixabay
Thank You for Choosing Sciक्षक ❤️

